Absorption is the process by which a drug moves from the site of administration into the bloodstream. The rate and extent of absorption depend on the environment where the drug is absorbed, its chemical properties, and the route of administration, which affects bioavailability. Routes of administration other than intravenous can result in incomplete absorption and reduced bioavailability.
Routes of Administration
Oral: Most drugs are erratically absorbed. They may undergo significant first-pass metabolism or be destroyed by stomach acid before absorption.
Sublingual: Absorption depends on the drug’s dilution; aqueous solutions are absorbed promptly. Bypasses first-pass metabolism, allowing rapid onset of action.
Intramuscular: Aqueous solutions are absorbed promptly, while depot preparations have slow and sustained absorption. Suitable for moderate volumes and irritating substances.
Subcutaneous: Similar to intramuscular absorption patterns; suitable for drugs that require slow release.
Inhalation: Rapid absorption, ideal for gases and localized treatment for respiratory conditions, minimizing systemic effects.
Topical: Absorption varies with skin condition and other factors; useful for localized treatment.
Transdermal (patch): Slow and sustained absorption; ideal for lipophilic drugs with poor oral bioavailability.
Rectal: Absorption is erratic and variable, partially bypassing first-pass metabolism. Useful when oral administration is not possible.
Mode of Absorption from the GI Tract
Drugs that are absorbed from the GI tract are usually absorbed through passive diffusion, facilitated diffusion, active transport, and endocytosis.
1. Passive Diffusion: Driven by concentration gradient, moving drugs from high to low concentration areas without the need for carriers. Most drugs are absorbed this way.
2. Facilitated Diffusion: Involves carrier proteins that help transport large molecules into cells. This process does not require energy but can be saturated and inhibited.
3. Active Transport: Uses energy (ATP) to move drugs against a concentration gradient. It is selective, saturable, and can be competitively inhibited.
4. Endocytosis and Exocytosis: Engulfs large drugs into the cell via vesicles (endocytosis) or releases substances from the cell (exocytosis). For example, vitamin B12 is absorbed through endocytosis, while neurotransmitters like norepinephrine are released by exocytosis.
Advantages and Disadvantages of Routes of Administration
Oral: Requires patient compliance; drugs may be metabolized before absorption.
Sublingual: Suitable for small doses; part of the dose may be lost if swallowed.
Intramuscular: Suitable for irritating substances; can be painful and requires aseptic technique.
Subcutaneous: Limited to small volumes; irritating drugs can cause pain or necrosis.
Inhalation: Effective for localized lung treatment; some patients may struggle with inhaler use.
Topical: Minimizes systemic absorption; not suitable for high molecular weight or poorly soluble drugs.
Transdermal: Ideal for lipophilic drugs; can cause irritation or delayed drug delivery.
Rectal: Suitable when oral administration is not possible; may irritate the rectal mucosa and is often not well accepted.
Examples
Oral: Amoxicillin
Sublingual: Nitroglycerin, Buprenorphine
Intramuscular: Vancomycin, Heparin, Haloperidol, Depot medroxyprogesterone
Subcutaneous: Epinephrine, Heparin
Inhalation: Albuterol, Fluticasone
Topical: Clotrimazole, Hydrocortisone cream, Timolol eye drops
Transdermal: Nitroglycerin, Nicotine, Scopolamine
Rectal: Bisacodyl, Promethazine
Factors affecting absorption of drugs:
1. Effect of pH on Drug Absorption: The absorption of drugs is influenced by their chemical nature, with most being either weak acids or weak bases. Acidic drugs (HA) can release a proton (H+), forming a charged anion (A-):
HA ⇌ H+ + A-
Weak bases (BH+) can also release a proton. However, the protonated form of basic drugs is typically charged, and losing a proton results in the uncharged base (B):
BH+ ⇌ B + H+
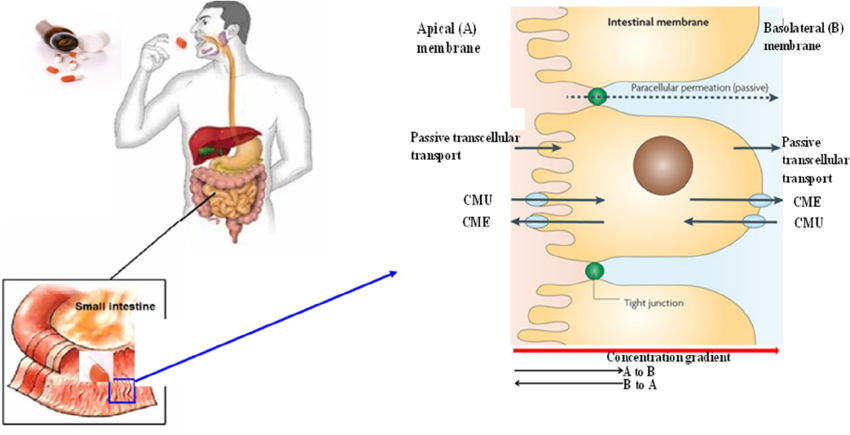
2. P-glycoprotein and Drug Transport: P-glycoprotein is a membrane transporter protein that moves various molecules, including drugs, across cell membranes (Figure 1.9). It is found in many tissues such as the liver, kidneys, placenta, intestines, and brain capillaries, and it plays a role in transporting drugs from tissues back into the blood. High expression of P-glycoprotein in certain areas decreases drug absorption because it actively pumps drugs out of cells.
Conclusion:
Drug absorption is a complex process influenced by various factors including the environment of absorption, the chemical properties of the drug, and the route of administration. Different routes of administration (oral, sublingual, intramuscular, subcutaneous, inhalation, topical, transdermal, and rectal) offer distinct advantages and disadvantages, impacting the rate and extent of drug absorption.
The mechanisms of absorption from the gastrointestinal tract include passive diffusion, facilitated diffusion, active transport, and endocytosis, each contributing differently to drug bioavailability. The pH of the absorption site and the presence of transport proteins like P-glycoprotein significantly affect drug absorption, with weak acids and bases undergoing specific ionization processes that influence their absorption and bioavailability.
Understanding these factors is crucial for optimizing drug delivery and ensuring effective therapeutic outcomes.
