Vancomycin-resistant Enterococcus faecium (VRE) presents a formidable challenge in healthcare due to its resistance to vancomycin, a potent antibiotic. VRE infections are primarily nosocomial, often affecting immunocompromised individuals or those with underlying health conditions. The emergence of VRE is linked to the overuse and misuse of antibiotics, leading to the selection pressure for resistant strains. Managing VRE infections requires a multifaceted approach, including strict adherence to infection control measures such as hand hygiene, environmental cleaning, and isolation protocols. Additionally, antibiotic stewardship programs aim to optimize antibiotic use to minimize the development and spread of antibiotic-resistant bacteria like VRE.
Quinupristin/dalfopristin, a combination drug of 30 parts quinupristin and 70 parts dalfopristin, is typically reserved for treating severe infections caused by vancomycin-resistant Enterococcus (VRE) when other treatment options are unavailable.
Mechanism of action:
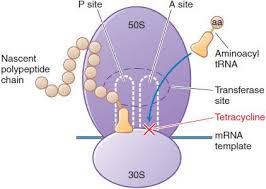
Both components of the drug bind to different sites on the bacterial ribosome. Dalfopristin disrupts the elongation phase of protein synthesis by inhibiting the addition of new amino acids to the peptide chain, while quinupristin prevents elongation, similar to macrolides, and causes the release of incomplete peptide chains. Together, they synergistically interrupt protein synthesis, exhibiting bactericidal activity against susceptible organisms with a prolonged post-antibiotic effect.
Antibacterial spectrum:
Quinupristin/dalfopristin primarily targets gram-positive cocci, including strains resistant to other antibiotics, with its main application being against E. faecium infections, particularly VRE strains. However, it is ineffective against E. faecalis.
Resistance:
Resistance mechanisms commonly involve enzymatic processes. For instance, ribosomal enzyme methylation interferes with quinupristin binding, potentially altering its bactericidal action to bacteriostatic. Dalfopristin can be inactivated by plasmid-associated acetyltransferase, while active efflux pumps reduce antibiotic levels in bacteria.
Therapeutic uses:
Quinupristin/dalfopristin is primarily used to treat severe infections caused by vancomycin-resistant Enterococcus (VRE), particularly E. faecium infections, when other treatment options are limited. It is effective against gram-positive cocci, including strains resistant to other antibiotics, making it a valuable option for combating infections that are difficult to treat with conventional therapies.
Pharmacokinetics:
Administered intravenously, quinupristin/dalfopristin does not achieve therapeutic concentrations via oral administration. It undergoes minimal metabolism and is primarily excreted in feces.
Quinupristin/dalfopristin is administered intravenously due to poor oral bioavailability. It does not undergo significant metabolism and is mainly excreted in feces, making dose adjustment unnecessary in patients with renal impairment. However, caution is advised in hepatic impairment due to potential accumulation.
The drug exhibits linear pharmacokinetics, with plasma concentrations proportional to the dose administered. Peak plasma levels are reached within 30 minutes to 1 hour post-infusion. Quinupristin/dalfopristin distributes widely into tissues, including lung, skin, and bone, achieving concentrations well above the minimum inhibitory concentration (MIC) for susceptible pathogens.
Its elimination half-life is approximately 1 to 1.5 hours, necessitating frequent dosing to maintain therapeutic levels. However, its prolonged post-antibiotic effect allows for less frequent dosing intervals.
Special considerations are warranted when administering quinupristin/dalfopristin. Due to its propensity to cause venous irritation, central line placement is preferred over peripheral administration. Additionally, monitoring for adverse effects such as hyperbilirubinemia and musculoskeletal symptoms is essential, especially with prolonged or high-dose therapy. Dose adjustments may be necessary when co-administering drugs metabolized by the cytochrome P450 CYP3A4 pathway to prevent potential drug interactions and toxicities.
Adverse effects:
Peripheral administration may cause venous irritation, necessitating central line use. Hyperbilirubinemia affects approximately 25% of patients due to competition with the antibiotic for excretion. Arthralgia and myalgia may occur with higher doses. Quinupristin/dalfopristin inhibits cytochrome P450 CYP3A4, potentially leading to drug toxicities when co-administered with drugs metabolized via this pathway.
Conclusion:
In conclusion, quinupristin/dalfopristin emerges as a valuable therapeutic option in combating infections caused by vancomycin-resistant Enterococcus (VRE), particularly E. faecium strains. Its unique mechanism of action, targeting protein synthesis through synergistic inhibition, makes it effective against gram-positive cocci, including strains resistant to other antibiotics. However, the emergence of resistance mechanisms underscores the importance of judicious antibiotic use and adherence to infection control measures.
While quinupristin/dalfopristin demonstrates linear pharmacokinetics with wide tissue distribution and prolonged post-antibiotic effect, careful consideration of administration routes and monitoring for adverse effects are crucial for optimizing therapeutic outcomes. As part of comprehensive antimicrobial stewardship efforts, quinupristin/dalfopristin serves as a critical tool in the management of VRE infections, highlighting the ongoing need for continued research and surveillance to address the evolving challenges posed by antimicrobial resistance in healthcare settings.
